Profile of the life science sector in the United Kingdom
The life science sector includes companies in the biotechnology, pharmaceutical, medical technology and medical diagnostics sectors. The term 'biopharmaceutical' refers to biotechnology and pharmaceutical companies.
What is a biotechnology company?
Also called biotech companies, biotechnology companies harness the process of organisms and living cells to create products to improve healthcare, agriculture, food and the environment. Such companies are most active in the healthcare sector where they are helping to generate more accurate and faster diagnostic platforms and to develop drugs that are more precise in their target and personalised treatments.
Drugs produced by biotech companies are commonly called biologics. Derived from living cells or through biological processes, such drugs are relatively complex molecules that are designed to replicate natural substances in our body. Some examples include hormones, vaccines, blood products, monoclonal antibodies, recombinant therapeutic proteins, gene and cellular therapies and growth factors. Where biologics have proven particularly beneficial is in the treatment of cancer, auto-immune diseases, genetic disorders and hard-to-treat diseases. But such medicines are expensive, because they need to be produced in living organisms which requires hundreds of process controls to assure predictable manufacturing. The structural complexity of biologics also makes their characterisation difficult and their clinical evaluation more challenging. Biologics also often need to be administered through infusions or injections which adds to the cost of such treatment.
In the context of food and agriculture, biotech companies are deploying the knowledge of science and genetics to make food cheaper to produce, longer lasting, more disease resistant as well as more nutritious. Biotech companies are also at the forefront of the development of biofuels, which involves generating alternative forms of fuel from plants and waste.
What is a pharmaceutical company?
Pharmaceutical companies research and develop and sell drugs typically manufactured through chemical synthesis. Such drugs generally have a simple chemical structure which means that their pharmacokinetics and pharmacodynamics are relatively predictable and amenable to simple dosing protocols for patients. In many cases they can be taken orally. Small molecule drugs make up 90 percent of the top prescribed medication of the market. Some common examples of such drugs include painkillers, like aspirin and paracetamol, and antihistamines, like diphenhydramine.
What is a medical technology company?
Medical technology companies develop, manufacture and sell devices, equipment and software designed to help with the diagnosis, treatment and care of patients. Found in hospitals, ambulances, doctors’ surgeries and even peoples’ homes, medical technology covers a very wide range of products. It includes general medical devices, like plasters and dressings for use on wounds, syringes, heart valves, ECG monitors, CT scanners and dialysis machines, as well as active implantable medical devices like cardiac pacemakers, cochlear implants and nerve stimulators. Medical technology also includes in vitro diagnostic devices used to examine specimens, such as pregnancy test kits, hepatitis B test kits and blood grouping reagents. Other forms of medical technology include surgical and laboratory equipment, sterile procedure packs, implants and prostheses as well as robots to help perform highly complex surgical operations. Digital tools and software also fall in the category of medical technology.
What are scientific supplies and service companies?
Laboratory and scientific equipment are essential to research and development within the biopharmaceutical and medical technology sector. Companies in this space include ones that are highly specialised and only produce one type of product or service, while others provide a multitude of services and products.
The wide variety of products offered by such companies falls into a number of categories. Firstly, there are those that provide general laboratory equipment, like sterile test tubes, flasks, petri dishes, pipettes, incubators, centrifuges, and microscopes as well as personal protective clothing like masks and gloves. Then there are the companies that supply a broad array of reagents, sold either individually or in ready-to-use kits. Such products include chemical substances, like solvents and acids, and biological products like antibodies, proteins, and cell lines. Other companies active in the space develop and supply instruments and reagents for carrying out DNA sequencing. Used in many different applications, the quality, strength and durability of each of these items are subject to strict control and evaluation.
Beyond companies supplying consumables, there are also those that provide services to biotechnology, pharmaceutical and medical technology companies on a contract basis. Known as contract organisations, these companies can help reduce the cost of having to do everything in-house. Contract companies vary in size and type of services they offer. The number of contract companies has increased over the past few years, fueled in part by the growing number of small companies who lack the full capacity to manufacture their products. Where CROs can help is in the formulation development, manufacturing and clinical testing of a drug or diagnostic, securing market approval as well as packaging and distribution.
Medical diagnostics
An important component of the healthcare system, medical diagnostics help to identify a patient’s medical condition or disease and can be used to monitor how well a treatment is working. While some diagnostic tests, such as those designed to detect pregnancy or to help in the management of diabetes, can be self-administered and the results read by the patient, others can only be performed in a clinical setting and need to be processed by a laboratory. There are many different types of medical diagnostics, which can be broadly divided between invasive and non-invasive tests.
Invasive diagnostic tests require cutting or entering a part of the body. Blood tests fall into this category as do biopsies, where tissue samples are taken from patients for analysis. Invasive diagnostics also include procedures that involve the insertion of medical instruments. One example is an endoscopy, where a long, thin tube with a small camera is put down the throat to visually examine the upper digestive tract. Another is a coronary angiogram, where a catheter is threaded through blood vessels to the heart from an artery in the groin, arm or neck to look at the structure and function of the heart.
Non-invasive diagnostics covers procedures that can be performed without breaking the skin or entering the body. One of the most common tests in this category is the X-ray, a technique that uses a form of electromagnetic radiation to see bones, tissues and structures inside the body. X-rays are often used to diagnose a broken bone or fluid in the lungs. Other non-invasive ways to see inside the body are computerised tomography, known as a CT scan, magnetic resonance imaging, or MRIs, and ultrasound scans.
Diagnostic tests can also be classified as in vivo and in vitro. The term in vivo comes from the Latin ‘within the living’ and refers to procedures performed in or on a living person. Some examples of in vivo diagnostics are the stethoscope and blood pressure devices. Coming from the Latin ‘in glass’ in vitro diagnostics (IVDs) are commonly performed in test tubes and similar equipment. Some of the most common and widely used in vitro diagnostics are clinical tests that analyse biological samples taken from the human body such as blood, saliva and urine. Such tests provide results based on either measurements of the concentration of specific substances or analytes like sodium and cholesterol, or by the detection of where a particular marker or set of markers are present or absent, such as genetic mutations or an immune response to an infection. All IVDs are subject to strict regulations.
Overview of the sector in 2020
Employment in the sector
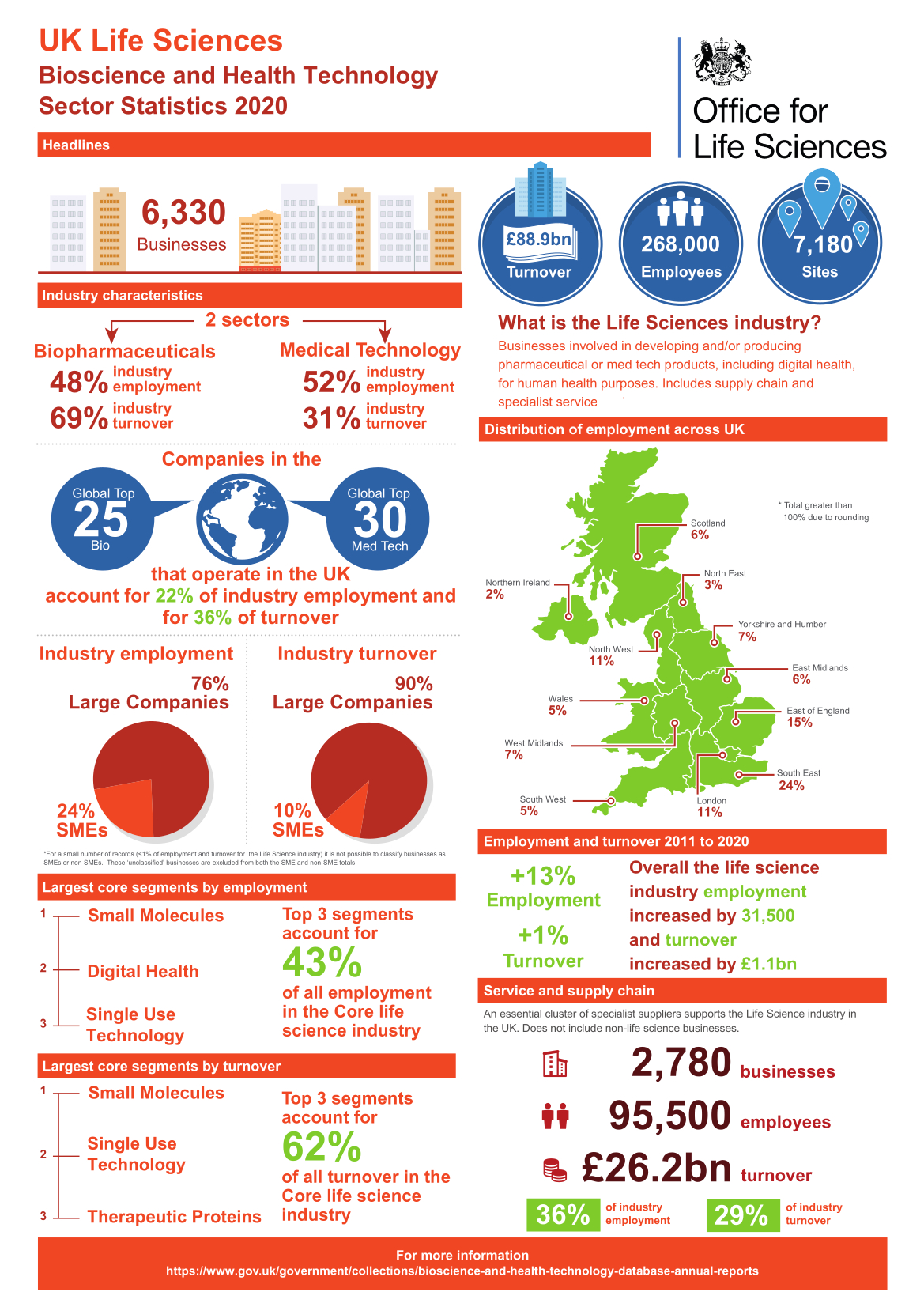
Businesses in the biopharmaceutical and medical technology sectors in the UK employed 282,023 people in 2021, a rise of 24% since 2009. There were 96,804 people employed at sites associated with research and development, 34% of all employees. The biopharmaceutical sector employs 48% of people in the sector with medical technologies companies employing the other 52%.
Employment in the life sciences in the UK 2009-2021
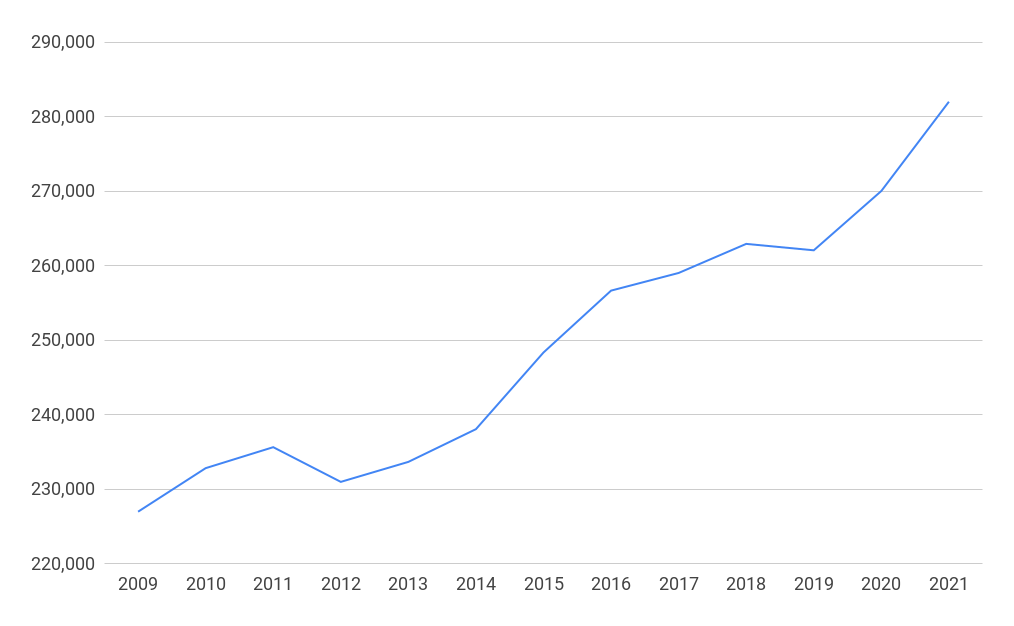
Employment at research and development sites in the life sciences in the UK 2009-2021
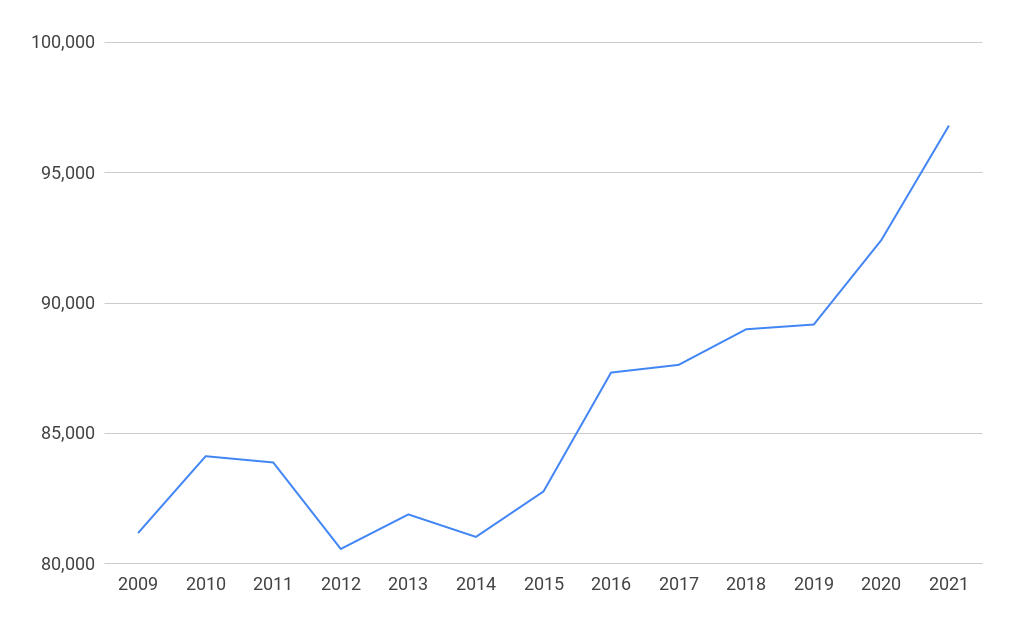
Employment in the life sciences in the UK by segment 2021
Employment by region 2021
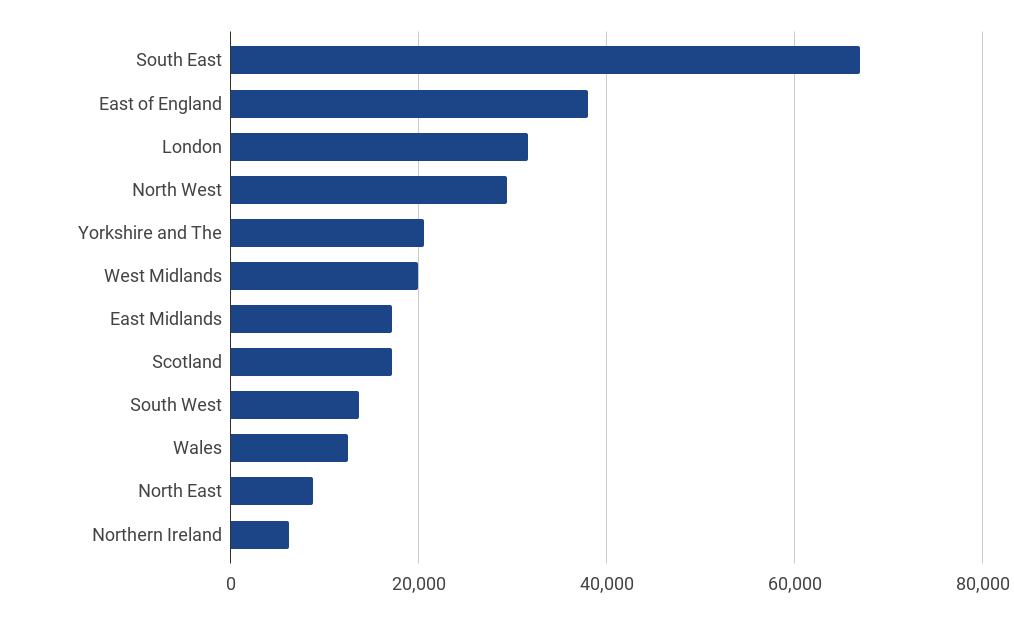
Businesses in the sector
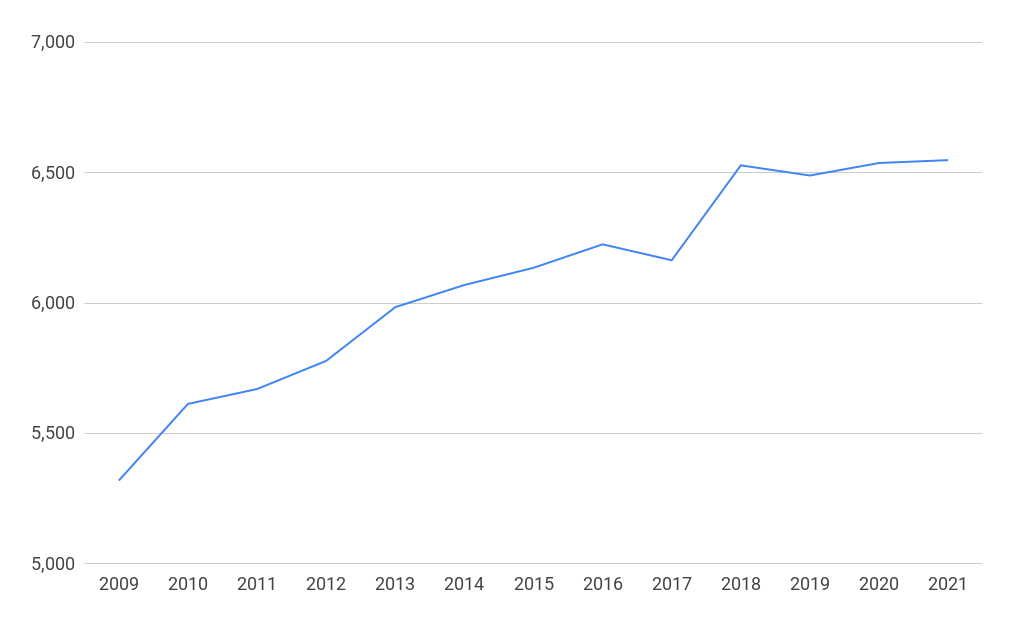
There were 6,548 businesses in the biopharmaceutical and medical technology sectors in the UK in 2021, a rise of 23% since 2009.
Businesses in the life sciences in the UK 2009-2021
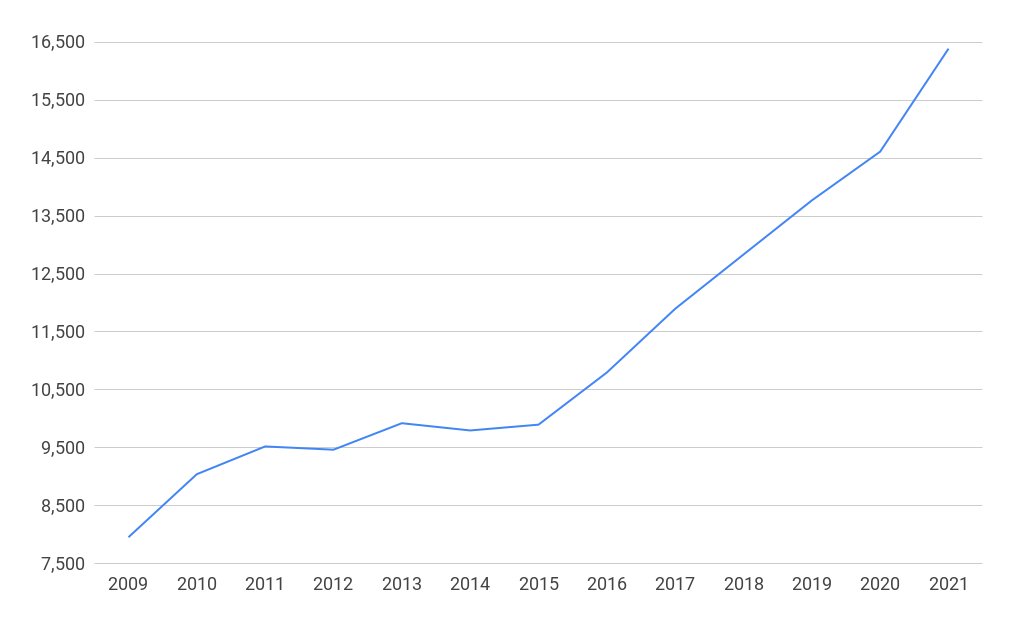
Digital health
There were 16,390 people employed in the digital health segment in the UK in 2021, a rise of 105% since 2009. The largest subsegments in digital health were hospital information systems with 4,936 employees and e-health - data analytics with 2,671 employees in 2021.
Employment in the digital health segment in the UK 2009-2021
Links
Association of British HealthTech Industries.
Association of the British Pharmaceutical Industry.
The Association for Clinical Biochemistry and Laboratory Medicine.
British Generic Manufacturers Association.
British In Vitro Diagnostics Association.
British Pharmacological Society.
British Society for Gene and Cell Therapy.
Digital Health & Care Alliance.